Thi Thanh Tam Nguyen,* Trung Hieu Do, Minh Thanh Tu, Tina Modjinou, Laurent Michely, Sena Hamadi, Daniel Grande, Thanh Binh Nguyen*
Macromolecules, 2025,
DOI: 10.1021/acs.macromol.5c00177
Abstract: As an abundant byproduct of hydrodesulfurization in petroleum refining processes, elemental sulfur has become a major environmental concern due to its costly disposal and challenging storage. However, the development of synthetic and processing methods to convert elemental sulfur into useful chemicals has not been widely investigated. In this study, we report the development of a novel multicomponent polycondensation (MCP) involving sulfur, diamines, and malonic acid under mild conditions to produce polythioureas. A family of 13 polythioureas with high structural diversity (seven homopolymers and six copolymers) is successfully synthesized from seven commercially available diamines, achieving high yields (up to 96%) and high molar masses (up to 115 900 g/mol). In particular, we report the first ever synthesis of a new biosourced polythiourea from naturally abundant vanillin with good yield upon applying this newly developed MCP.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Quang Huy Tran, Cao Nguyen Nguyen, Ngoc Lan Chu, Thai Thanh Thu Bui, Thi Thu Tram Nguyen, Minh Tu Ha,* Dinh Hung Mac,* Thanh Binh Nguyen*
Chem. Select, 2025, 10, e202501419
DOI: 10.1002/slct.202501419
Abstract: A switchable chalcogenation of 2,3-dihydrofurans with elemental sulfur or molecular oxygen to construct fully substituted thiophenes or furans was developed. In this version of thiophene generation, the sulfuration occurs through a cascade of sulfurative ring editing with inexpensive sulfur powder. Conversely, the production of furans is enabled by aerobic oxidation in basic media.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Le Anh Nguyen, Quoc Anh Ngo,* Pascal Retailleau and Thanh Binh Nguyen*
Chem. Commun. 2024, 60, 13586-13589.
Abstract: 2-Tetralones were found to undergo dithiocarbamation with elemental sulfur and isothiocyanates S8/R–N=C=S in the presence of N-methylpiperidine as a base catalyst under solvent-free conditions. The reaction could proceed quickly at room temperature to provide convenient access to substituted 4-hydroxythiazolidine-2-thiones with complete atom efficiency. The adducts could be easily dehydrated in neat TFA to give thiazole-2-thiones.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Le Anh Nguyen, Thi Thu Tram Nguyen, Quoc Anh Ngo,* Pascal Retailleau and Thanh Binh Nguyen*
Org. Biomol. Chem. 2024, 22, 9353-9356.
DOI: 10.1039/d4ob01452a
Abstract. An efficient method to construct 4-phenyl-4-hydroxyquinazolin-2-thiones 3 via solvent-free and catalyst-free addition of o-aminobenzophenone 1 with aryl isothiocyanates 2 under thermal conditions has been developed, providing new congested 4-phenyl-4-hydroxyquinazolin-2-thiones 3 in practically quantitative yields via simple purification by filtration/recrystallization. Extension of these conditions to o-aminoacetophenone 4 in place of o-aminobenzophenone 1 led to 4-methylenequinazoline-thione 5 as a result of a subsequent dehydration reaction.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Le Anh Nguyen, Dinh Hung Mac,* Pascal Retailleau and Thanh Binh Nguyen*
Org. Biomol. Chem. 2024, 22, 8586-8590.
DOI: 10.1039/D4OB01512F
Abstract. The elemental sulfur and isothiocyanate (S8/RNCS) couple was found to undergo redox condensation with o-halonitrobenzenes in the presence of N-methylpiperidine as a base in N-methylpyrrolidin-2-one
to provide step- and redox-economical access to 2-aminobenzothiazoles.
Alternatively, dithiocarbamate salts generated in situ from addition of amines and CS2 could be used in place of the S8/RNCS couple.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Le Anh Nguyen, Thi Yen Tran, Quoc Anh Ngo,* Dinh Hung Mac, Pascal Retailleau, Thanh Binh Nguyen*
Org. Lett. 2024, 26, 6098-6102.
DOI: 10.1021/acs.orglett.3c02835
Abstract. We disclose the synthesis of 3-arylquinoxalin-2-ones from o-phenylenediamines and readily available arylacetates. The method harnesses the selective oxidative property of elemental sulfur in the presence of amine base catalyst and DMSO. The reactions are operationally simple and tolerate a wide range of functional groups.

xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Duc Long Le, Thi Yen Tran, Le Anh Nguyen,* Quoc Anh Ngo,* Dinh Hung Mac, Thanh Binh Nguyen*
Asian J. Org. Chem. 2024, 13, e202400212.
DOI: 10.1002/ajoc.202400212
Abstract. A method to assemble 2-aminobenzoxazoles via iron-catalyzed redox condensation of amines, carbon disulfide and 2-nitrophenols is reported. The reaction features advantages of environmentally friendly iron catalyzed conditions, readily available starting materials, and excellent atom-, step- and redox- economy compared to known protocols. Experimental investigation suggests a mechanism encompassing iron-catalyzed reduction of the nitro group of 2-nitrophenols by the dithiocarbamate group from amine-CS2 adduct.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx Thi Thanh Nhan Vu, Thu Ngoc Trinh, Thu Trang Pham, Minh Tu Ha, Dinh Hung Mac,* Pascal Retailleau and Thanh Binh Nguyen*
Adv. Synth. Catal. 2024, 366, 2691-2695.
DOI: 10.1002/adsc.202400304
Abstract. Tetrasubstituted 2,3-trans-dihydrofuran and furan are important heterocyclic scaffolds in natural product, bioorganic and medicinal chemistry as well as in material science. The synthesis of both of these heterocycles starting from common and readily available starting materials are challenging. We found that in situ generated deoxybenzoin-chalcone Michael adducts underwent oxidative annulation upon heating with molecular iodine in DMSO to provide selectively 2,3-trans-dihydrofurans at 80 °C and furans at 120 °C.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Cao Nguyen Nguyen, Duc Toan Nguyen, Ha An Tran, Hung Dinh Mac,* Thi Thu Tram Nguyen, Pascal Retailleau and Thanh Binh Nguyen*
Org. Biomol. Chem. 2024, 22, 3871-3875.
Abstract. A cost-effective, practical, straightforward and scalable synthesis of α-pyrones via base- and sulfur-promoted annulation of phenylacetates and chalcones. Generated in situ from the starting components by using dbu as a base catalyst, the Michael adducts underwent a smooth oxidative cyclization into 3,4,6-triaryl-2-pyranones upon heating with dabco and sulfur in DMSO. Extension to malonate in place of phenylacetates led to 4,6-diaryl-2-pyranone-2-carboxylates.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Minh Hang Nguyen, Hai Sam Nguyen, Le Anh Nguyen, Bich Ngoc Nguyen, Hai Thuong Cao, Dinh Hung Mac* and Thanh Binh Nguyen*
Eur. J. Org. Chem. 2024, e202301319.
DOI: 10.1002/ejoc.202301319
Abstract. In the presence of a
base catalyst (20 mol %) such as DBU, DABCO or Na2S•3H2O/N-methylpyrrolidone, phenylacetylenes
were found to efficiently undergo sulfurative amination with elemental sulfur
and anilines under neat conditions, leading to N-aryl thioamides with complete atom efficiency.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Duc Long Le, Le Anh Nguyen,* Ngoc Binh Vo, Thi Thu Tram Nguyen, Quoc Anh Ngo,* Pascal Retailleau and Thanh Binh Nguyen*
Org. Biomol. Chem. 2024, 22, 1167-1171.
Abstract. Inexpensive sodium sulfide trihydrate was found to promote unprecedented 6e- regio-predefined redox condensation of o-nitroanilines with α-tetralones to benzo[a]phenazines. The method was also successfully extended to acetophenones and higher homologs as the reducing partners to provide 2-phenylquinoxalines. Compared to traditional approaches to benzo[a]phenazine and quinoxaline cores starting with o-phenylenediamines, the present strategy could afford these heterocycles with well-defined regiochemistry based on the structure of starting o-nitroanilines.
 xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Supasorn Phaenok, Le Anh Nguyen, Darunee Soorukram, Thi Thanh Tam Nguyen, Pascal Retailleau, Thanh Binh Nguyen*
Chem. Eur. J. 2024, e202303703.
DOI: 10.1002/chem.202303703
Abstract. Thiourea derivatives are in-demand motifs in organic synthesis, medicinal chemistry and material science, yet redox methods for the synthesis that start from safe, simple, inexpensive and readily available feedstocks are scarce. In this article, we disclose the synthesis of these motifs using elemental sulfur and nitromethane as the starting materials. The method harnesses the multi-electron auto-redox property of nitromethane in the presence of sulfur and amines, delivering thiourea products without any added oxidant or reductant. Extension of this reaction to cyclizable amines and/or higher homologues of nitromethane led to a wide range of nitrogen heterocycles and thioamides. Operationally simple, the reactions are scalable, tolerate a wide range of functional groups, and can be employed for the direct functionalization of natural products. Mechanistically, the nitro group was found to act as an oxidant leaving group, being reduced to ammonia whereas sulfur, along with the role of a sulfur building block for the thiocarbonyl group, behaved as a complementary reductant, being oxidized to sulfate.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Le Anh Nguyen, Supasorn Phaenok, Duc Long Le, Thi Thu Tram Nguyen, Quoc Anh Ngo*, and Thanh Binh Nguyen*
Org. Lett. 2023, 25, 5145-5150.
Abstract. As frequently encountered byproducts of isocyanate chemistry, hydrogen sulfide and related sulfur containing compounds should be treated in a safe way to lower their adverse health and environmental effects, especially in large scale syntheses. As a proof of concept, we report herein an example of in situ recycling of sulfur byproduct to reductant in the synthesis of bioactive 2-aminobenzoxazoles 3. Using an Fe/S catalytic system, this heterocyclic scaffold could be obtained from o-nitrophenols 1 with isothiocyates 2 via direct redox condensation consisting of reduction of the nitro group of 1 by the sulfur moiety of 2.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Bich Ngoc Nguyen, Minh Hieu Tran, Thai Thanh Thu Bui, Dinh Hung Mac*, Van Phong Pham*, Pascal Retailleau, and Thanh Binh Nguyen*
J. Org. Chem. 2023, 88, 15, 11197-11204.
Abstract.
Elemental sulfur and DABCO were found to be an excellent combination to
promote a one-pot cascade of condensation-oxidative cyclization of
chalcones and unsubstituted cyanoacetamide in DMSO to provide
3-cyanopyrid-2-ones.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxHoang Yen Nguyen, Thi Minh Chau Tran, Van Ha Nguyen, Pascal Retailleau, Dinh Hung Mac* and Thanh Binh Nguyen*
Org. Biomol. Chem. 2023, 21, 503-507.
Abstract. 1-Anilinonaphtho[2,1-b]thiophenes could be conveniently synthesized from a three-component reaction of 1-acetonaphthones with anilines and elemental sulfur under catalyst-free simple heating conditions.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Thanh Binh Nguyen* and Pascal Retailleau
Chem. Commun. 2022, 58, 13333-13336.
Abstract. Thieno[3,4-b]thiophene cores could be conveniently obtained via one-pot sulfurative tetramerization of acetophenones using a ternary system comprised of elemental sulfur, a base and DMSO under simple heating conditions. The structure of the products was verified by X-ray crystallography.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Thi Thu Tram Nguyen, Viet Dung Duong,Thi Ngoc Nga Pham, Quoc Thanh Duong and Thanh Binh Nguyen*
Org. Biomol. Chem. 2022, 20, 8054-8058.
Abstract.The elemental sulfur–DMSO couple was found to efficiently promote the oxidative coupling of active methylhetarenes with amines to yield amides under simple heating conditions. When 2-methylquinoline was used as the methylhetarene component, the formation of the expected 2-quinolinecarboxamides from anilines could be efficiently catalyzed by iron, nickel and cobalt salts. The method displayed good functional group tolerance and was applicable to aromatic, heteroaromatic and aliphatic amines. Other substrates such as phenylacetic acid, dibenzyl disulfide, and benzylamine could act as competent partners in place of methylhetarenes.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Thanh Binh Nguyen,* and Pascal Retailleau
Org. Lett. 2022, 23, 6676-6680.
DOI:10.1021/acs.orglett.2c02736
Abstract.2-Naphthols 1were found to react with aryl isothiocyanates 2 to provide 2-iminonaphtho-1,3-oxathioles 3 in the presence of DABCO as a catalyst. When elemental sulfur was used, moderate to good yields of 3 could be achieved from a nearly equimolar mixture of two starting materials 1 and 2. Products 3 could also be formed in the absence of sulfur or an external oxidant. In the latter case, an additional equivalent of 2 was found to act as a dehydrogenating agent.
 xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Thanh Binh Nguyen,* Hung Dinh Mac,* Thi Minh Chau Tran, Bich Ngoc Nguyen and Hai Thuong Cao
Org. Biomol. Chem. 2022, 20, 7226-7231.
DOI: 10.1039/D2OB01343F
Abstract.3-Arylquinoxaline-2-thiones were conveniently synthesized via three-component oxidative condensation of acetophenones with o-phenylenediamines and sulfur in DMSO in the presence of piperidine as a catalyst. The products could be readily isolated from the reaction mixture by simple precipitation and washing with methanol. This set of reaction conditions applied to higher homologs of acetophenones as well as benzyl phenyl ketones led to 2,3-di-C-substituted quinoxalines. Further functionalization of 3-phenylquinoxaline-2-thione via reaction on the thione group could be readily performed to provide quinoxaline derivatives in good yields.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Le Anh Nguyen, Thi Thu Tram Nguyen, Quoc Anh Ngo,* Thanh Binh Nguyen*
Adv. Synth. Catal. 2022, 364, 2748-2752.
DOI: 10.1002/adsc.202200527
Nguyen, L. A.; Nguyen, T. T. T.; Ngo, Q. A.; Nguyen, T. B. Adv. Synth. Catal. 2022, 364, 2748-2752.
Abstract. While elemental sulfur has been largely used as oxidant or sulfurating agent, its role as a catalyst has not been developed. We report here its catalytic activity in the oxidative condensation of o-phenylenediamines with acetophenones in DMSO to provide quinoxalines. The method was also extended to α-tetralones, propiophenones (R=Me) as well as higher homologues (R=Et, n-Pr) in place of acetophenones, leading to a wide range of naphthoquinoxalines and 3-substituted 2-arylquinoxalines.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx Nang Duy Lai, Thu Trang Nguyen, Nhu Ngan Ha Nguyen, Pascal Retailleau, Dinh Hung Mac* and Thanh Binh Nguyen*
DOI: 10.1039/D2QO00526C
Org. Chem. Front. 2022, 9, 3163-3168.Abstract. 3-Cyanothiophene is an important heterocyclic scaffold in bioorganic and
medicinal chemistry as a useful synthetic intermediate as well as in
materials science as a privileged motif for photovoltaic development.
Herein, we report our unexpected results on the formation of
3-cyanothiophene derivatives as the major products via a
three-component reaction of chalcones, benzoylacetonitriles and
elemental sulfur along with the minor products 2-aminothiophenes. The
ratios between these two thiophene products is 4 : 3 and could be varied
by simply changing the promoting base as well as its stoichiometric
ratio. The method was successfully extended to benzoylacetate in place
of benzoylacetonitrile to provide thiophene-3-carboxylates.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx Thanh Binh Nguyen*
Nguyen, T. B. Clean Technol. 2022, 4, 234-238.
DOI: 10.3390/cleantechnol4020013
Abstract. Elemental sulfur (S8) was found to react very rapidly (<1
min) with a stoichiometric amount of triphenylphosphine at rt in
sufficient amount of solvent (0.2–0.5 mL of solvent/1 mmol of PPh3).
Compared to the previously described methods, the present procedure
constitute excellent access to triphenylphosphine sulfide.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Tan Sang Truong, Pascal Retailleau, Thanh Binh Nguyen*
Asian J. Org. Chem. 2022,11, e202100751
DOI: 10.1002/ajoc.202100751
Truong, T. T.; Retailleau, P.; Nguyen, T. B. Asian J. Org. Chem. 2022, 11, e202100751
Abstract.
TFA/DMSO combination was found to efficiently promote the
cross-dehydrogenative coupling of a wide range of hetaryl thiols with
indole derivatives, leading to 3-(hetarylsulfenyl)indoles in good-to
excellent yields under mild heating conditions. Two-fold
hetarylsulfenylation of indoles in 2- and 3- positions could be
conveniently achieved by simply adapting molar ratio of the starting
materials.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx Thanh Binh Nguyen,* and Pascal Retailleau
Org. Lett. 2021, 23, 5344-5348.
DOI:10.1021/acs.orglett.1c01653
Nguyen, T. B.; Retailleau, P. Org. Lett. 2021, 23, 5344-5348.
Abstract. A convenient synthesis of thiazole-2-thiones was developed based on base-catalyzed three-component reac-tions between chalcones, isothiocyanates and elemental sulfur.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Thanh Binh Nguyen,* Dinh Hung Mac,* and Pascal Retailleau
J. Org. Chem. 2021, 86, 9418-9427.
DOI: 10.1021/acs.joc.1c00740
Nguyen, T. B.; Mac, D. H.; Retailleau, P. J. Org. Chem. 2021, 86, 9418-9427.
Abstract. While Gewald reaction was well known for more than haft a century as an excellent method providing bioactive 2-aminothiophenes from reactions of α-cyanoacetates and carbonyl compounds and elemental sulfur, its application to dibenzoylmethanes as the carbonyl substrates was however unknown and experimentally proven unsuccessful. We proposed here a convenient approach to such series of compounds by a DABCO catalyzed, one-pot, two-step, three-component reaction of α-cyanoacetate with chalcones and elemental sulfur. This catalytic strategy is highlighted by its excellent atom/step efficiency and high degree of structural diversification by simply choosing the suitable starting chalcones, which are unarguably much more readily available than dibenzoylmethanes.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Le Anh Nguyen, Thi Thu Tram Nguyen, Quoc Anh Ngo,* Thanh Binh Nguyen*
Org. Biomol. Chem. 2021, 19, 6015-6020.
DOI:10.1039/D1OB00976A
Nguyen, L. A.; Nguyen, T. T. T.; Ngo, Q. A.; Nguyen, T. B. Org. Biomol. Chem. 2021, 19, 6015-6020.
Abstract. An Fe/S catalyst generated in situ from FeCl2·4H2O and elemental sulfur S8 in the presence of a tertiary amine as a base was found to catalyze efficiently a 6e− redox condensation of o-nitrophenols
with acetophenones and methylquinolines. The condensed products
2-benzoylbenzoxazoles and 2-quinolylbenzoxazoles were obtained in
reasonable yields with water as the only byproduct at a temperature as
low as 80 °C.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Le Anh Nguyen, Quoc Anh Ngo,* Pascal Retailleau, Thanh Binh Nguyen*
Adv. Synth. Catal. 2021, 363, 2098-2103.
DOI:10.1002/adsc.202100150
Nguyen, L. A.; Ngo, Q. A.; Retailleau, P.; Nguyen, T. B. Adv. Synth. Catal. 2021, 363, 2098.
Abstract. Although compounds with a 2-benzoylbenzoxazole motif are biologically relevant, there are only a few methods for synthesizing them, most of which relied on multistep process or required substrates bearing activating groups. Herein, we report an efficient method for the synthesis of such compounds by direct reactions of o-aminophenols with acetophenones promoted by sulfur in DMSO. The reaction was found to proceed via a Willgerodt rearrangement-type benzoxazolation of acetophenones followed by a benzylic oxidation to reinstall the carbonyl function. This method has a broad substrate scope and good tolerance for sensitive functional groups.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Thi Thu Tram Nguyen, Le Anh Nguyen, Quoc Anh Ngo,* Marina Koleski and Thanh Binh Nguyen*
Org. Chem. Front. 2021, 8, 1593.
DOI: 10.1039/D0QO01654C
Nguyen, T. T. T.; Nguyen, L. A.; Ngo, Q. A.; Koleski, M.; Nguyen, T. B. Org. Chem. Front. 2021, 8, 1593-1598.
Abstract. Thioamides could be conveniently synthesized in good to excellent yields via DMSO-promoted oxidative coupling of methylhetarenes with amines in the presence of a near stoichiometric amount of sulfur (1.25 equiv.). Both aliphatic and aromatic amines were found to be competent substrates. When anilines o-substituted by cyclizable groups such as OH, NH2, NHPh, SH and CONH2 were used as amine substrates, the corresponding hybrid bis-aza-heterocycles were formed in high yields even with a sulfur loading as low as 0.5 equiv.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Thanh Binh Nguyen,* Dinh Hung Mac,* and Pascal Retailleau
J. Org. Chem. 2020, 85, 13508-13516.
DOI: 10.1021/acs.joc.0c01618
Nguyen, T. B.; Mac, D. H.; Retailleau, P. J. Org. Chem. 2020, 85, 13508.
Abstract. Chalcones were found to undergo sulfurative dimerization with elemental
sulfur to tetrasubstituted 1,3-dithioles. The reaction was found to
proceed at room temperature in the presence of a nitrogen-base catalyst
in DMSO.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Thanh Binh Nguyen,* and Pascal Retailleau
Adv. Synth. Catal. 2020, 362, 5380-5384.
DOI: 10.1002/adsc.202000964
Nguyen, T. B.; Retailleau, P. Adv. Synth. Catal. 2020, 362, 5380.
Abstract. Strong Brønsted acids were found to catalyze the oxidative condensation of 2-aminothiophenols with aryl alkyl ketones in DMSO to provide a wide range of complex 2-arylbenzothiazines. With acetophenones, dimeric 2-arylbenzothiazines were formed. On the other hand, the reaction with propiophenones resulted in 2:1 adducts whereas 1:1 adducts were produced with isobutyrophenones, benzoylacetonitrile and ethyl benzoylacetate.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
J. Org. Chem. 2020, 85, 12058.
Nguyen, T. M.; Cao, H. A.; Cao, T. T. T.; Koyama, S.; Mac, D. H.; Nguyen, T. B. J. Org. Chem. 2020, 85, 12058.
Abstract. [2,1]Benzothiazine S,S-dioxides 2 were synthesized by simply heating o-nitrostyrenes
with elemental sulfur in 3-picoline with complete atom economy. This
reaction was found to occur without any added catalyst and consist of a
cascade of reduction of the nitro group, sulfuration of a C–H of the
double bond, oxidation of a sulfur atom to its highest oxidation state
by the migration of two oxygen atoms from the nitro group, and formation
of new N–S bonds. Furthermore, the method could also be applied to o-nitrocinnamamides and cinnamate esters.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Thanh Binh Nguyen,* and Pascal Retailleau
Adv. Synth. Catal. 2020, 362, 3824.
DOI: 10.1002/adsc.202000725 Nguyen, T. B.; Retailleau, P. Adv. Synth. Catal. 2020, 362, 3824.
Abstract. In the presence of TFA as a strong acid catalyst in DMSO, α,α’‐enolizable ketones were found to be stereoselectively α,α’‐functionalized with 2‐aminothiophenols to provide spirobis(1,4‐benzothiazine) derivatives.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Thanh Binh Nguyen*
Adv. Synth. Catal. 2020, 362, 3448.
DOI: 10.1002/adsc.202000535 Nguyen, T. B. Adv. Synth. Catal. 2020, 362, 3448.
Abstract. It is well known that heterocycles are among the most significant molecules for everyday life, ranging from natural products and bioactive substances to functional materials. This review will focus on the synthesis of heterocycles by reactions involving elemental sulfur published from 2017 until now.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Le Anh Nguyen, Thai Duy Dang, Quoc Anh Ngo,* Thanh Binh Nguyen*
Eur. J. Org. Chem. 2020, 3818.
DOI: 10.1002/ejoc.202000523 Nguyen, L. A.; Dang, T. D.; Ngo, Q. A.; Nguyen, T. B. Eur. J. Org. Chem. 2020, 3818.
Abstract. Oxidation with sulfur: Elemental sulfur (S8) was found to be
an excellent stoichiometric oxidant to promote oxidative condensation of
2‐aminophenols with a wide range of aldehydes to provide benzoxazoles.
Chemistry views
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Thanh Binh Nguyen,* Tuan Minh Nguyen, Pascal Retailleau
Chem. Eur. J. 2020, 26, 4682
DOI: 10.1002/chem.201905597
Nguyen, T. B.; Nguyen, T. M.; Retailleau, P. Chem. Eur. J. 2020, 26, 4682.
Abstract. In this study we reported on a very simple technique to perform efficiently photodimerization of some vinylpyridines. By irradiating a stirred mixture of several stilbazoles with solid oxalic acid dihydrate dispersed in a non‐polar (e. g. cyclohexane) or moderately polar (benzene, dichloromethane, dioxane) solvent, the corresponding dimeric cyclobutane adducts were obtained in high yields with excellent of regio‐ and stereo‐ selectivities. The strategy could also be applied successfully to oily, waxy or even insoluble stilbazoles. Moreover, the oxalic acid loading could be lowered to sub‐stoichiometric amounts. When further optimizations were needed, our strategy was found to be highly flexible to identify other oligocarboxylic acids as alternative additive to improve or even overturn regioselectivity. Oxalic acid and other oligocarboxylic acids were found to be capable of orienting more than fifty stilbazoles toward photodimerization under these conditions.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Thi Thu Tram Nguyen, Van Anh Le, Pascal Retailleau, and Thanh Binh Nguyen*
Adv. Synth. Catal. 2020, 362, 160.
DOI: 10.1002/adsc.201901235
Nguyen, T. T. T.; Le, V. A.; Retailleau P.; Nguyen, T. B. Adv. Synth. Catal. 2020, 362, 160.
Abstract. A straightforward access to 2‐amino‐3‐arylthiophenes has been developed via one‐pot two‐step three‐component reaction of arylacetonitriles, chalcones and elemental sulfur. The first step consists of a DBU‐catalyzed formation of Michael adduct between arylacetonitriles and chalcones. The second step is a cascade of DABCO‐catalyzed sulfuration of the Michael adduct with elemental sulfur followed by an oxidative cyclization to afford thiophenes. Compared to the Gewald reactions and related transformations which are limited in acetonitriles bearing a methylene group activated by an α‐substituted electron withdrawing group as substrates, our method can be applied to a wide range of arylacetonitriles and requires only catalytic amounts of DBU and DABCO. The developed reaction opens an access to 3‐aryl‐2‐aminothiophenes complementary to classical Gewald reactions with high degree of structural diversity and atom efficiency.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Thanh Binh Nguyen,* Le Anh Nguyen, and Pascal Retailleau
Org. Lett. 2019, 21, 6570
DOI: 10.1021/acs.orglett.9b02558.
Nguyen, T. B.; Nguyen, L. A.; Retailleau P. Org. Lett. 2019, 21, 6570.
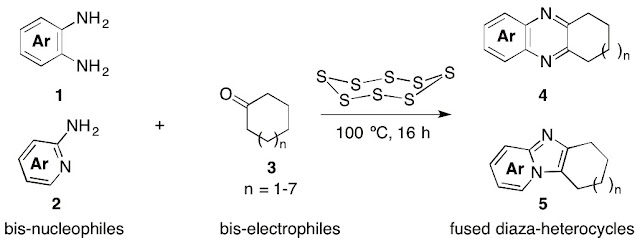
Abstract. The S8/DMSO combination was found to be an excellent oxidizing agent for ipso,α,β,γ-tetramination of cyclohexanones with two molecules of o-phenylenediamines in the presence of TFA as a catalyst. The strategy could be applied to pyrido cyclohexanes as a direct method to extend the fused heteroaromatic system.
Thi Thu Huong, Le, Chitose Youhei, Thanh Binh Nguyen* and Dinh Hung Mac*
Org. Biomol. Chem. 2019, 17, 6355.
Abstract. An efficient synthesis of 2H-3-nitrothiochromenes via a cascade reaction was established. Starting from commercially available o-bromobenzaldehydes and β-nitrostyrenes with sodium sulfide nonahydrate as an inexpensive sulfur source, various substituted thiochromenes were synthesized with high functional group tolerance without any added transition metal catalyst or additive.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Thanh Binh Nguyen,* Hou Jing-ya, and Pascal Retailleau
Adv. Synth. Catal. 2019, 361, 3337.
DOI: 10.1002/adsc.201900371
Nguyen, T. B.; Retailleau P. Adv. Synth. Catal. 2019, 10.1002/adsc.201900371
Abstract. A sulfur-promoted three-component reaction of isatoic anhydride, primary aliphatic or aromatic amines, and acetophenones leading to densely substituted 3-substituted 2-aroylquinazolin-4(3H)-ones is reported. The key step involves a cascade reaction of selective oxidation of the methyl group of the acetophenones, followed by a condensation with anthranilamides. The scope of the reaction is applicable to the synthesis of tryptanthrin and various 3-unsubstituted 2-aroylquinazolin-4(3H)-ones.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Thanh Binh Nguyen* and Pascal Retailleau
Adv. Synth. Catal. 2019, 361, 3588.
DOI: 10.1002/adsc.201900436
Nguyen, T. B.; Retailleau P. Adv. Synth. Catal. 2019, 10.1002/adsc.201900436

Abstract. Sulfur was found to be an excellent catalyst for the oxidative coupling of cyclohexanones with o-aminophenols using DMSO as oxidant. A wide range of heteropropellanes were obtained with complete regio and stereoselectivities and functional group tolerance.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Thanh Binh Nguyen* and Pascal Retailleau
J. Org. Chem. 2019, 84, 5907.
DOI: 10.1021/acs.joc.9b00408
Nguyen, T. B.; Retailleau P. J. Org. Chem. 2019, DOI: 10.1021/acs.joc.9b00408
Abstract. By simply heating phenylacetonitriles 1 with elemental sulfur and DMSO in the presence of a catalytic amount of DABCO, we have performed an oxidative trimerization leading to polysubstituted pyrrole heterocycles 2 in excellent yields and E-configuration.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Le Anh Nguyen, Pascal Retailleau and Thanh Binh Nguyen*
Adv. Synth. Catal. 2019, 361, 2864.
DOI: 10.1002/adsc.201900160
Nguyen, L. A.; Retailleau P.; Nguyen, T. B. Adv. Synth. Catal. 2019, DOI: 10.1002/adsc.201900160
Abstract. Malonic acid derivatives could be conveniently prepared with high degree of functional flexibility via redox condensation reactions between anhydride maleic, amines, elemental sulfur and DMSO as oxidant. This multicomponent decarboxylative transformation consists in a cascade of ring opening, decarboxylative oxidative thioamidation at temperature as low as 50 °C.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Thanh Binh Nguyen,* Le Phuong Anh Nguyen and Thi Thu Tram Nguyen
Adv. Synth. Catal. 2019, 361, 1787.
DOI: 10.1002/adsc.201801695
Nguyen, T. B.; Nguyen, L. P. A.; Nguyen, T. T. T. Adv. Synth. Catal. 2019, DOI: 10.1002/adsc.201801695
Abstract. In the presence of catalytic amounts of elemental sulfur, dibenzyl disulfide/DMSO was found to be an excellent thiobenzoylating agent of amines to provide a wide range of thioamides. The reaction becomes autocatalytic when anilines substituted by an o‐cyclizable group were used as nucleophile, leading to the corresponding 2‐aryl aza heterocycles.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
59. Sulfur-Promoted Decarboxylative Sulfurative Hexamerization of Phenylacetic Acids: Direct Approach to Hexabenzylidyne Tetrasulfides
Thanh Binh Nguyen* and Pascal Retailleau
Org. Lett. 2019, 21, 279.
DOI: 10.1021/acs.orglett.8b03728
Nguyen, T. B.; Retailleau, P. Org. Lett. 2019, 21, 279.
Abstract. During our experiments aimed at understanding the reaction pathways by
which arylacetic acids were oxidatively decarboxylated and condensed
with different nucleophiles in the presence of elemental sulfur, these
acids have been treated with sulfur powder in dimethyl sulfoxide (DMSO)
and N-methylpiperidine in the absence of nucleophiles, producing a
remarkable symmetrical sulfurated hexamer consisting of six benzylidyne
moieties and four sulfur atoms.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
58. Sulfur-Promoted Aminative Aromatization of 1,2,3,4- Tetrahydrophenazines with Amines: Flexible Access to 1- Aminophenazines
Thanh Binh Nguyen* and Pascal Retailleau
Adv. Synth. Catal. 2018, 360, 2389.
DOI: 10.1002/adsc.201800260
Nguyen, T. B.; Retailleau, P. Adv. Synth. Catal. 2018, 360, 2389.
Abstract. Elemental sulfur was found to be an excellent reagent for promoting the aminative aromatization of 1,2,3,4-tetrahydrophenazines with amines to provide a wide range of functionalized 1-aminophenazines.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
M. Q. Tran, T. B. Nguyen, W. R. Sawadogo, L. Ermolenko, S. Song, P. Retailleau, M. Diederich, A. Al-Mourabit, Eur. J. Org. Chem. 2018, 5878.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Thanh Binh Nguyen, Le Anh Nguyen, Mathilde Corbin, Pascal Retailleau, Ludmila Ermolenko, and Ali Al-Mourabit,
Eur. J. Org. Chem. 2018, 5861.
DOI: 10.1002/ejoc.201701607
Nguyen, T. B.; Nguyen, L. A.; Corbin, M.; Retailleau, P.; Ermolenko, L.; Al-Mourabit, A. Eur. J. Org. Chem. 2018, 5861.
Abstract. By simply switching solvents, two sets of conditions for total regio- and stereo- selective photodimerization of (E)-3-(imidazo[1,2-a]pyrimidin-2-yl)acrylic acid (E)-7a were found. In acetonitrile in the presence of benzophenone as a photosensitizer or in cyclohexane, head-to-head cis-trans-cis isomer 8a was the only observable cycloadduct. In DMSO, head-to-head trans-trans-trans isomer 10a was obtained as the unique cycloadduct. The diester 9a was cyclized into the challenging fully substituted diimidazobenzobutane 13 system present in benzosceptrins.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Thanh Binh Nguyen* and Pascal Retailleau
Green Chem. 2018, 20, 387.
DOI: 10.1039/C7GC03437G
Nguyen, T. B.; Retailleau, P. Green Chem. 2018, 20, 387.
Abstract. A sulfurative self-condensation method for constructing thiophenes 2 by reaction between ketones 1 with elemental sulfur is reported. The reaction, which is catalyzed by anilines and their salt with strong acids, starts from readily available and inexpensive materials, and releases only water as a by-product.
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
 CONTACT:
CONTACT:































































































![[BOND]](https://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) H Ketimines and Applications
H Ketimines and Applications


